
Heated Air Pressure Massager: Policy Trends and Market Opportunities in the Wellness Industry
In early 2025, federal policy discussions have centered on safety and quality in the wellness industry, including Heated Air Pressure Massager.
As the global focus shifts toward preventive health and at-home wellness care, the Heated air pressure massager is emerging as a key solution in both consumer and medical-grade recovery devices. Simultaneously, health authorities around the world are introducing new policies to ensure these innovations meet standards of safety, sustainability, and functionality.
In this article, we explore regulatory updates, global policy landscapes, and market pathways for Heated air pressure massager products. We also share insights from FOFOMEDICAL (https://www.fofomedical.com), a company at the forefront of smart wellness innovation.
Evolving Global Health Device Policies
In the post-pandemic era, medical and wellness device regulations are evolving rapidly. Countries are introducing frameworks to manage devices that blend therapeutic effects with consumer use — including Heated air pressure massager products.
Key global regulatory developments include:
-
WHO is advancing a framework for “connected medical devices,” urging manufacturers to integrate privacy, safety, and risk controls from the design phase.
-
Some countries are reclassifying wearable heat and air-pressure devices into medium-risk categories, requiring clinical safety evaluations and post-market surveillance.
These changes indicate a clear direction: Heated air pressure massager products must now be backed by robust compliance, product lifecycle monitoring, and sustainability commitments.
Wholesale Heated Air Pressure Massager
Contents
- 1 United States: Federal and State-Level Frameworks
- 1.1 European Union and UK: New Classifications and UDI Tracking
- 1.1.1 Asia-Pacific: Local Validation for Expanding Markets
- 1.1.2 Consumer awareness and safety education remain vital. Labels include warnings for use in pregnancy, cardiovascular conditions, and pacemaker implants — aligning with FDA and MDR guidelines.
- 1.1.3 Sustainability in Packaging and Lifecycle Design
- 1.1.4 FOFOMEDICAL’s Global Vision for Innovation and Safety
- 1.1.5 Looking Ahead: Future Trends in Heated Air Pressure Technology
- 1.1 European Union and UK: New Classifications and UDI Tracking
United States: Federal and State-Level Frameworks
In the United States, Heated air pressure massager devices typically fall under FDA Class II designations and must go through the 510(k) clearance process, demonstrating substantial equivalence to approved devices.
As of 2025, new FDA guidance includes:
-
Heat exposure limits capped at 42°C to prevent thermal injury.
-
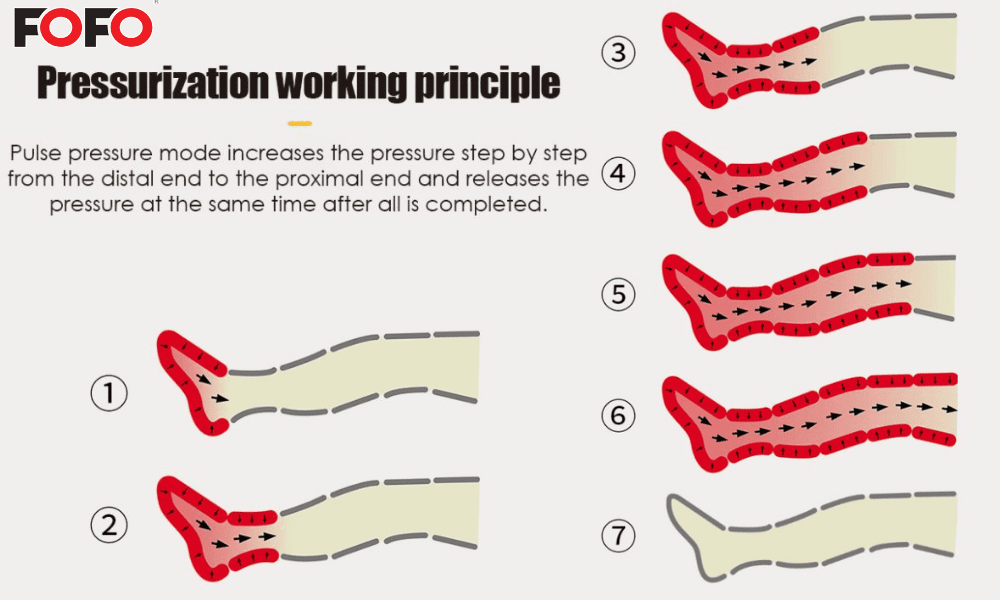
Compression pressure maximums under 300 mmHg, with requirements for programmable safety cycles.
-
Smart devices must meet updated energy efficiency and digital health standards.
State-level policies are also becoming influential:
-
California now requires all health and wellness electronics to carry energy usage labeling.
-
New York mandates recyclable packaging and device materials with verified environmental claims.
For Heated air pressure massager producers, this dual-layer of regulation means harmonizing safety engineering with regional compliance — particularly in packaging, emissions, and recyclability.
European Union and UK: New Classifications and UDI Tracking
With the EU MDR now fully in effect, Heated air pressure massager products are often classified as Class IIa devices under Rule 21. That means:
-
Mandatory clinical evaluation reports and post-market performance tracking.
-
Unique Device Identification (UDI) systems must be embedded for traceability.
-
Upload of product and performance data into EUDAMED (the EU’s device database) is required.
In the UK, UKCA marking is replacing CE for medical devices. Though similar in many ways to MDR, UK guidance now includes:
-
Separate safety verification for firmware or over-the-air updates.
-
Enhanced cybersecurity assessments for any device with app connectivity.
FOFOMEDICAL successfully completed MDR compliance in early 2025 by adopting modular architecture, enabling separate certification of heating and pressure control units while maintaining cohesive system functionality.
Hot Sale Heated Air Pressure Massager
Asia-Pacific: Local Validation for Expanding Markets
In Asia, Heated air pressure massager devices are seeing increased consumer demand driven by aging populations and recovery-focused lifestyles.
China’s NMPA classifies these products under Category II devices and requires:
-
Local biocompatibility and pressure endurance testing.
-
Clinical data from domestic users under supervised trials.
-
Product registration that includes labeling in Simplified Chinese.
Japan’s PMDA allows fast-track approval via third-party certification (CAB), but requires localized usability studies and continuous post-market data monitoring.
Asia-Pacific compliance focuses on localization: multilingual labeling, culturally-adapted documentation, and responsive after-sales support are key to success.
Compliance-Driven Product Strategy for Heated Air Pressure Massager
At FOFOMEDICAL, innovation is driven by both consumer wellness needs and evolving compliance standards. Our Heated air pressure massager product line is built on these four strategic pillars:
-
Precision Temperature Control: PID algorithms maintain heat within 1°C variance, preventing thermal injury.
-
Sustainable Materials: Replaceable TPU airbags and recyclable PC-ABS casings reduce electronic waste.
-
Smart Data Systems: Wi-Fi and Bluetooth connectivity allows real-time monitoring, cloud-based reporting, and app-integrated control.
-
Global Compliance Network: Dedicated compliance teams monitor regulatory changes in the U.S., EU, and Asia to keep products aligned with dynamic standards.
Consumer-Focused Features: Wellness Meets Usability
Heated air pressure massager devices serve a diverse audience — from post-exercise recovery users to seniors managing chronic fatigue or poor circulation.
Key features include:
-
Customizable Programs: App-based control lets users design massage and heat cycles tailored to their needs.
-
Human-Centric Ergonomics: Adjustable angles and skin-safe fabrics ensure comfort during extended use.
-
Silent Operation: Quiet air pump technology keeps noise below 45 dB, suitable for office or nighttime use.
-
Visual Feedback: LED displays and mobile app graphs show live data on temperature, pressure, and usage time.
Consumer awareness and safety education remain vital. Labels include warnings for use in pregnancy, cardiovascular conditions, and pacemaker implants — aligning with FDA and MDR guidelines.
Sustainability in Packaging and Lifecycle Design
Environmental compliance is now part of medical wellness design. To meet global green policies, FOFOMEDICAL integrates:
-
Eco-Packaging: 90% FSC-certified cardboard, soy-based ink, and compostable inner packaging.
-
Low Power Consumption: Sleep-mode energy usage under 0.3W, qualified for EU A+++ efficiency labels.
-
Modular Design: Replaceable massage heads and battery units reduce waste and extend product life.
These steps not only comply with state and national mandates but also reflect consumer expectations for brands that prioritize ESG performance.
Heated Air Pressure Massager
FOFOMEDICAL’s Global Vision for Innovation and Safety
As a global provider of smart rehabilitation and wellness technology, FOFOMEDICAL brings unique strengths to the Heated air pressure massager segment:
-
Dual Protection System: Thermal sensors and air pressure feedback loops ensure safe operation in real time.
-
AI Learning Engine: Devices analyze thousands of user patterns to optimize heat-pressure cycles and personalize user experience.
-
Blockchain Traceability: From raw material sourcing to customer feedback, every device is trackable — meeting FDA and MDR post-market reporting requirements.
-
Global Service Network: Service centers in North America, Europe, and Asia ensure localized diagnostics, replacements, and 24/7 support.
Looking Ahead: Future Trends in Heated Air Pressure Technology
As the health-tech sector expands, Heated air pressure massager devices are likely to evolve in several directions:
-
Connected Wellness: 5G-enabled devices will sync with smartwatches and health apps, allowing personalized recovery plans.
-
Eco-Innovations: Biodegradable polymers and graphene heating elements may replace current synthetics, reducing environmental impact.
-
Multi-Scenario Integration: From under-desk massage mats to travel-friendly leg wraps, products will serve broader use cases.
Policy support for connected health, sustainable materials, and eldercare solutions will fuel further innovation in this space.
The journey of the Heated air pressure massager from a niche wellness tool to a globally regulated therapeutic device reflects the evolving intersection of health, policy, and technology. While compliance demands continue to grow across the U.S., Europe, and Asia, they also create a framework for safety, innovation, and consumer trust.
FOFOMEDICAL remains committed to meeting these challenges with innovation-first product development, sustainable practices, and robust regulatory alignment. By collaborating with policymakers, consumers, and health professionals, we are shaping the future of wellness technology — one safe, smart, and eco-conscious device at a time.
As awareness of home therapy tools increases, so too will the expectation for responsible product design and policy alignment. The Heated air pressure massager market stands at the crossroads of comfort and compliance — and with the right strategy, it can thrive in both.
Previous News
FOFO to Showcase Advanced Medical Solutions at ...Next News
Policy driven innovation of sputum suction mach...Feature Product
-
Portable Electric Sputum Suction Machine FO9001-D
Portable Electric Sputum Suction Machine ...
-
Phlegm Suction Machine – Electric & ...
Electric Surgical Portable Phlegm Suction...
-
Portable Compressor Nebulizer – Dog Design BC68...
Portable Compressor Nebulizer – Dog Design: A L...



